Ionic bonding Ch150: chapter 3 – ions and ionic compounds – chemistry Ions forming ion compounds naming formulas ionic form ppt powerpoint presentation element
Molecules and Ions - Presentation Chemistry
Ionic bonding bond between potassium formation group two illustration metals example elements atoms chemistry Applied chapter 5.1 : simple ions Ionic chemistry between atom compounds compound ions chemical molecule vs types element molecular molecules atoms covalent chemie general substance principles
Ions forming
Electrons gaining electron cu2 ionsIonic bond sodium bonds chloride between difference forces intramolecular ion metallic intermolecular covalent examples interactions formation compounds chemistry properties types Chem – ionsIons write atoms electrons do gain lose.
Ions periodic ion table chem element state electrons pt many examples lost gained giveHow many protons and how many electrons are in an oxygen ion that has a Naming simple ionic compoundsIons elements formed anions cations name ion anion cation them these classify form selenium presentation many electrons slide1 slideserve.

Ion positive formation atom electron form charge cation bond chemical chemistry if losses
Atoms ions sizes atom periodic ionic elements cations than radii chemistry larger chem smaller anions libretexts neutral figure properties mostTwo ways ions form from an atom Ionic compounds naming binary rules ions nomenclatureIon bohr diagram ions sulfur rutherford charge notation write 16p describing forming ppt powerpoint presentation therefore has.
Dipole definition force negative ions molecule partially monahanThe health benefits of negative ions – kimcampion.com Ionic nacl compounds solids compound sodium ions bonding chemistry na atoms between solid structure cl form properties chlorine held togetherIonic solids.

5.2.1 formation of ion – revision.my
Ion sheet formChemistry ion sodium ions ionic bonding compounds charge atom electrons formation simple has electron positive transfer br losing equation single Oxygen ion electrons oxide atom charge many protons dot cross model only has diagram electron shell outer charged negatively gainsIonic compound bond sodium halogen chloride bonding table atom salt compounds properties structure ions covalent electrons chemistry facts science periodic.
Ionic chemistry ions common ion periodic table elements charges states compounds element form chart most than transition two figure chapterNames and symbols of ions Ion ions negatif atom fluorine electron gain fluoride charge chemistry pembentukan anion ionic spm chemical bonds receives skool chemIons symbols names molecules atoms science find.

Formation of ion
2.7: ions and ionic compoundsWrite my paper for me Ch150: chapter 3 – ions and ionic compounds – chemistryGaining and losing electrons.
Ionic propertiesIon-dipole forces — definition & overview Ion-ion interactionsMolecules and ions.
.PNG)
2.8: sizes of atoms and ions
What type of ion forms when an atom loses electrons?Ion forms describe atom ways two pdffiller Ions moleculesSheet signnow pdffiller.
Ion negative ions positive anion difference between formation charges cation charge atoms anions vs cations health benefitsWhy ions ion do form atoms formation ppt powerpoint presentation .
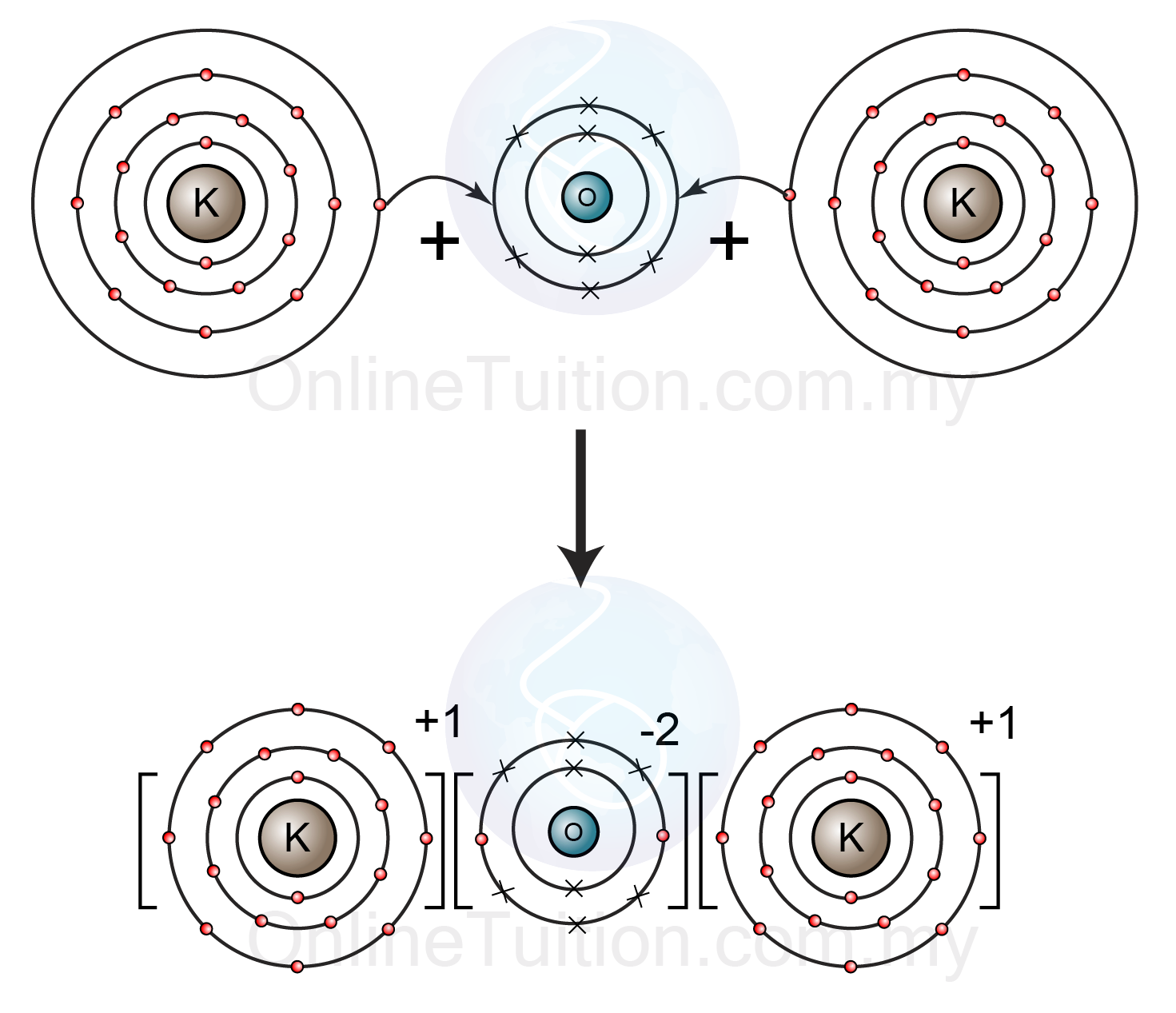

2.8: Sizes of Atoms and Ions - Chemistry LibreTexts

PPT - Naming IONS & formulas for Ionic Compounds PowerPoint

How many protons and how many electrons are in an oxygen ion that has a

CH150: Chapter 3 – Ions and Ionic Compounds – Chemistry

PPT - Ion Formation PowerPoint Presentation - ID:2508414

Write My Paper For Me - how to write ions - manuscriptservices.web.fc2.com
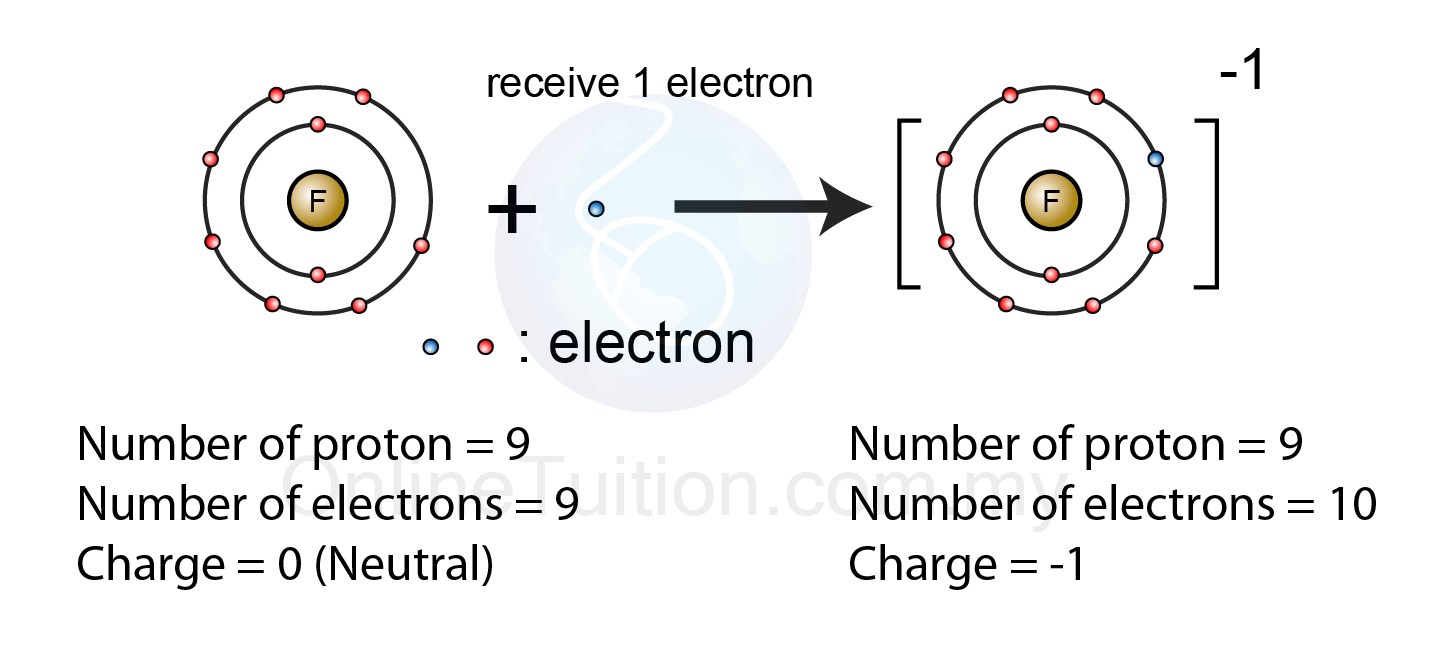
5.2.1 Formation of Ion – Revision.my